first published: 08.07.2021
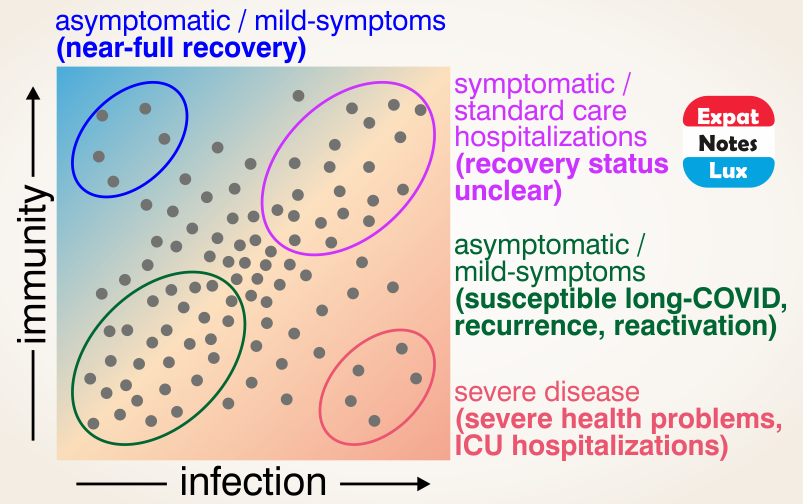
Do we have any insights on HOW some people get ‘long-COVID’ post-infection?
This is a very intriguing question and while there is no clear mechanistic detail available yet to make the distinction between why some people get neurological symptoms like loss of smell or taste post SARS-CoV-2 infection and others do not, here is an early hypothesis on how some people might get neurological and other manifestations post COVID-19.
In a recent study, researchers from UK used hamster brain slices to show that the SARS-CoV-2 Spike-protein receptor binding domain (RBD) can induce a constriction in blood flow in blood capillaries (very fine blood vessels which brings red-blood cells to the organs in the body) evoked by pericytes. Pericytes are specialized multi-functional cells which wrap around the endothelial cells (a thin line of cells inside the capillary) and help maintain the homeostatic (steady state of physical and chemical conditions) and hemostatic (process to prevent and stop bleeding) functions, and effectively plays a cruitial role in the maintenance and sustainance of the blood–brain barrier.
Angiotensin-converting enzyme 2 (ACE2) is a primary receptor for SARS-CoV-2 entry into human cells and is highly expressed in pericytes, and a target for SARS-CoV-2 as well. Moreover, a mutated RBD (which cannot bind to ACE2) does not show constriction in blood-flow, showing that the effect is most likely mediated through the ACE2 proteins on the pericyte cell-surface.
In terms of the restricted (reduced) blood flow to human brain, it is expected that neuronal functions would be impaired, and in extreme cases (heavy or long-term blood restriction), neuronal cells can even die. Effectively meaning, imparing of neuronal functions like smell or taste (short term) and in case of neuronal cell deaths, permanent or long-COVID as well (neurons once mature, do not replicate and hence any neurological manifestation would be permanent). Similarly, in heart, cardiac pericytes (also has high ACE2 expression) can explain acute heart injuries to certain degree, and in case of kidney pericytes, it might explain the multi-organ failures as well (data not available, only hypothesis)
It is quite early to make the above conclusions and is fully ackowledged that the hypothesis is very limited in scientific accuracy, and more evidences and correct mechanistic details would emerge in time. And it is also expected that an additional layer of mechanism would be in play as these effects are not seen in everyone infected but only few people (more affected than the others). In other words, the why is still under investigation and currently not known, but the above hypothesis seems like a first link in the chain of events that would probably explain how the ‘long-COVID’ manifests.
- High ACE2 expression levels in pericyte cells, Journal: scientific data, first published: 21.08.2018, title: Single-cell RNA sequencing of mouse brain and lung vascular and vessel-associated cell types (PDF file, 3.9Mb). The ACE2 expression levels in different brain cells can be accessed at Christer Betsholtz group at the Uppsala university database as well.
- ACE2 highly expressed in heart pericytes, Journal: bioRxiv, first published: 12.05.2020, Pre-Print (not-peer reviewed), title: Pericyte-specific vascular expression of SARS-CoV-2 receptor ACE2 – implications for microvascular inflammation and hypercoagulopathy in COVID-19 patients (PDF file, 30.9Mb)
- SARS-CoV-2 crosses the blood–brain barrier, Journal: Nature Neuroscience, first published: 16.12.2020, title: The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice (PDF file, 5.3Mb)
- Long-term damage and changes in brain post COVID-19, Journal: Journal of Clinical Investigation, first published: 25.02.2021, title: Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations (PDF file, 9.8Mb)
- Pericytes in cardiac complications post SARS-CoV-2 infection, Journal: American Journal of Physiology-Heart and Circulatory Physiology, first published: 01.11.2020, title: Role of angiotensin-converting enzyme 2 and pericytes in cardiac complications of COVID-19 infection (PDF file, 1.9Mb)
- Pericyte-mediated brain blood-capillary constriction, Journal: bioRxiv, first published: 01.04.2021, Pre-Print (not-peer reviewed), title: SARS-CoV-2 binding to ACE2 triggers pericyte-mediated angiotensin-evoked cerebral capillary constriction (PDF file, 915kb)
- Reduced renal blood flow in acute kidney injury post COVID-19, Journal: Shock, first published: 01.04.2021, title: Critically Ill COVID-19 Patients With Acute Kidney Injury Have Reduced Renal Blood Flow and Perfusion Despite Preserved Cardiac Function: A Case-Control Study Using Contrast-Enhanced Ultrasound (abstract ONLY, full article not freely available)